Information & Resources for Medical Professionals
Is ColoTest® right for your patients? Learn more about this OTC screening option for colorectal cancer.
What is ColoTest®?
ColoTest® is a FIT test, the preferred annual stool screening method for colon cancer according to Colorectal Cancer Alliance and Cleveland Clinic.1 ColoTest® detects blood in stool, which may be an early warning for:
Colorectal Cancer, Diverticulitis, Gastrointestinal Disorders, Colitis and Polyps.
ColoTest® advantages include:
- Fast Results as Soon as 1 Minute
- Self Test at Home
- No Lab Testing Required
- Only 1 Stool Sample 2
- No Diet Restrictions
- 98.8% Accurate
2 Bleeding in the digestive systems may be intermittent (not continuous). If concerned, it is recommended to perform the test on three different days with new feces samples each time to increase the chance of detecting fecal occult blood.
Colon Cancer Stats


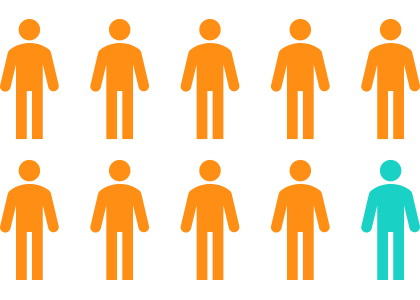
Screening Saves Lives
- More than 29 million Americans are NOT screening for Colon Cancer.4 Given the statistics above, we really want them to get screened.
- Screening is typically recommended from age 45 to 75, but earlier screening may be desired depending on family health history.
- Early onset colorectal cancer is a growing concern, with the proportion of cases among those younger than 55 years increased from 11% in 1995 to 20% in 2019.5
ColoTest® provides one of the easiest and least invasive screening options available to your patients, but there are many options available as outlined below. Consider engaging with your patients to determine their screening status and encourage them to consider screening if they are out of compliance.

- 1 https://pubmed.ncbi.nlm.nih.gov/36856579/
- 2 https://www.cancer.org/cancer/types/colon-rectal-cancer/about/key-statistics.html
- 3 https://www.ucsfhealth.org/education/colorectal-cancer-prevention-and-screening
- 4 https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
- 5 https://acsjournals.onlinelibrary.wiley.com
There are several options available for colorectal cancer (CRC) screening. Screening methods recommended by the Colorectal Cancer Alliance are outlined below.

- Sensitivity-true positives – this percentage indicates the number of people WITH colorectal cancer that are correctly identified
- Specificity-true negative – this percentage indicates the number of people WITHOUT colorectal cancer that are correctly identified
- 1 https://pubmed.ncbi.nlm.nih.gov/36856579/
- 2 https://www.goodrx.com/conditions/colon-cancer/colonoscopy-cost
- 3 https://www.goodrx.com/conditions/colon-cancer/at-home-colon-cancer-test
- 4 https://consultqd.clevelandclinic.org/colorectal-cancer-screening-choosing-the-right-test/
ColoTest® Kit Contents:
One Quick
Guide
One
Insert
One Test Casette in a Sealed Foil Pouch
One Sample Collection Tube
One Collection Paper
1. Prepare Stool Specimen:
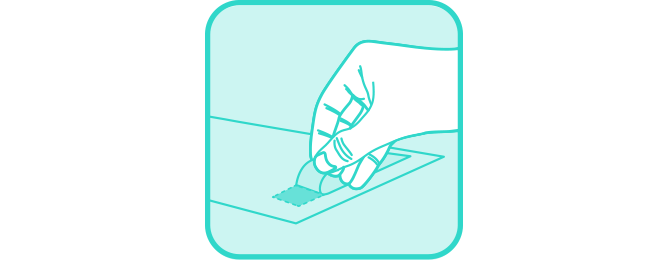
Remove tape covers from back sides of collection paper.
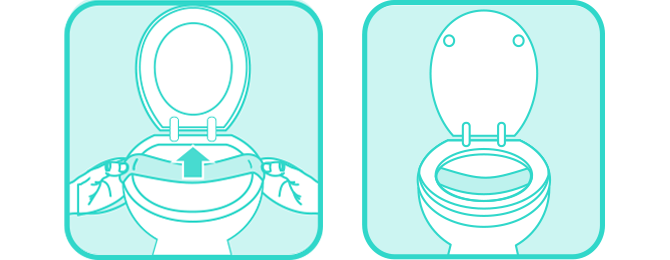
2.
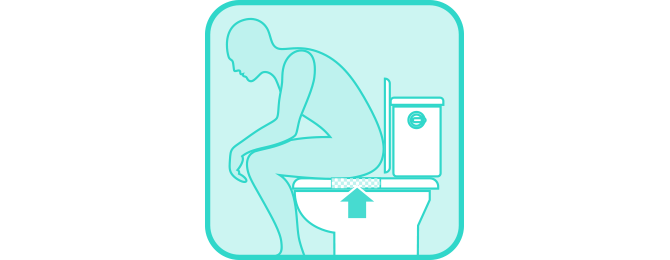
Loosely place paper at the back portion of the toilet bowl, affix tape, and then lower the seat.
3.
Deposit stool specimen on collection paper.

4.
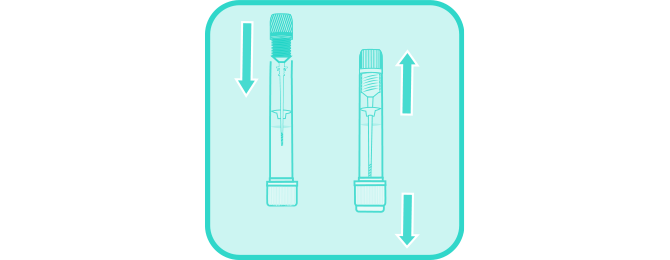
Unscrew and remove sampler from the collection tube.

5.
Using the grooved tip of sampler, pierce the stool in at least 5 different sites.
6.
Insert sampler with specimen back into collection tube, firmly tighten, and shake the tube to mix the liquid. Flush the remaining stool and paper.
2. Perform the Test:

7.
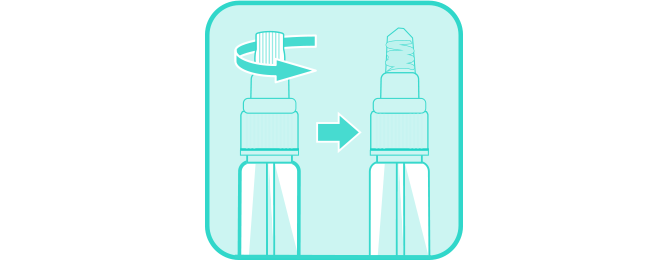
Open sealed foil pouch, remove test cassette, and place on a flat surface.

8.
DO NOT remove cassette cap from device
9.
Holding the collection tube upright, unscrew the clear cap.
10.
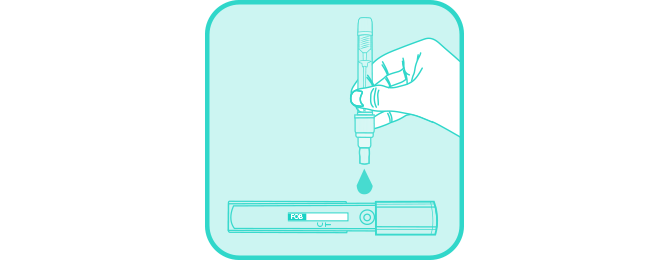
Squeeze the collection tube to deliver one drop of the sample into the sample well as shown.


11.
Close the cap with force until it stops to ensure reaction
Within 1-10 minutes, read the result window of the cassette.
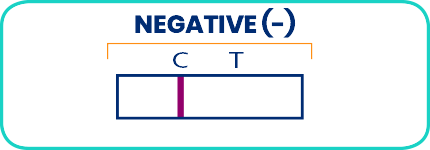
Follow the reading instructions below to identify whether your result is positive, negative or invalid. For positive results, consult a doctor.

Two lines in the result window: The specimen contains a detectable level of blood.
Many conditions may cause blood in your stool. Contact your doctor right away.

How Does ColoTest® Work?
The test is a Driven Flow chromatic immunoassay. The device consists of one test strip in a plastic cassette. The test strip consists of:
- A conjugate pad treated with mouse anti-human hemoglobin antibodies conjugated with colloidal gold.
- A strip of nitrocellulose membrane with a Test line (T-line) and a Control line (C-line). The T-line is coated with anti-hHb antibodies, and the C-line is coated with goat anti-mouse IgG antibodies.
When an adequate volume of test specimen is dispensed into the sample well of the cassette, the test specimen migrates across the test strip. If the concentration of hHb in the specimen is at or above 50 ng/mL, the T-line visibly appears as a burgundy line. The intensity of the T-line may vary according to the concentration of the hHb in the sample. If the concentration of hHb in the sample is below 50 ng/mL, a T-line does not visibly appear. The C-line is coated with goat anti-mouse IgG antibody, which binds to the conjugated monoclonal antibody regardless of whether hHb is present in the sample. Assay reading time is 1-10 minutes.


0-23513-13214-3
0-23513-13215-0
Where is ColoTest® Available?
ColoTest® can be ordered by the pharmacist through the following wholesalers:








